It’s a sunny day on the campus of the University of California, Santa Barbara, but little light penetrates the labs and offices of Shuji Nakamura. Shades sheathe the windows in part because, Nakamura says, “I worry about unknown people around here—that they will include a spy.”
That sounds farfetched until you consider that Nakamura, who played a lead role in the development of blue light-emitting materials, is now back for a second act. In the 1990s Nakamura gained fame by cooking up the first semiconductor materials to emit bright blue light—a boon for displays and data storage—and sparked a global race to perfect the materials. He made those trailblazing lasers and their glowing cousins, light-emitting diodes (LEDs), at Tokushima, Japan-based Nichia—and became a sort of national folk hero in the process. (When Nakamura is in Tokyo, subway riders accost him for his autograph.)

Photo 1. White-light LEDs
Now, having moved to an academic post in the United States, he is at it again—caught up in an intense worldwide competition. That’s because the bright blue emitting devices that are the progeny of his original inventions provide a key stepping stone in a high-stakes effort to produce white-light LEDs that are sufficiently cheap, pleasing, and efficient to crush Edison’s almighty light bulb—and radically transform the $40 billion general-illumination industry.
Briskly crossing a courtyard, Nakamura enters a semiconductor test room, and grad student John Kaeding hands him a plastic container bearing a translucent Oreo-size disc. Nakamura sits on a stool and touches a test electrode to a spot near the disc’s center. The disc is made of sapphire and coated with at least 30 invisibly thin layers of materials whose fundamental workings are not fully understood but whose properties astonish. Instantly, a bluish-green glow emanates. Nakamura lifts the electrode and touches a spot closer to the disc’s edge; it shines a grassy green. A third spot is dimly aquamarine; a fourth, violet; a fifth, bright blue—the color needed to create a solid-state device that produces white light.

Photo 2. Shuji Nakamura
Nakamura appears pleased, yet bright as this blue is, he knows immediately that it’s not bright enough. That’s why his grad students spend balmy California nights slaving over the 1,000 °C ovens in which these materials are grown, tweaking chemistries and temperatures, changing substrates, altering flow rates of gases. “We try, try, try,” Nakamura says. A breakthrough, he adds, “could come in three years; could come today or tomorrow.” But let there be no mistaking his goal: “I want to replace all the conventional lighting.”
He’s not the only one. Dozens of companies and academic groups like Nakamura’s Solid State Lighting and Displays Center are feverishly seeking to produce a new kind of lighting by developing bright white LEDs (see table 1). Indeed, just as transistors replaced vacuum tubes, LEDs promise to replace today’s glass-encased incandescent and fluorescent light bulbs: LED lights use far less electricity than an average light bulb, and they shine for a far longer time before burning out.
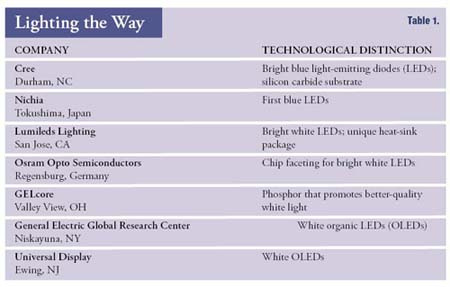
Table 1
Most researchers are, like Nakamura, pursuing LEDs made of such semiconducting materials as gallium nitride; a few other groups are developing more exotic organic light-emitting diodes (OLEDs), which are like plastic sheeting and herald the day when softly glowing patches of material replace ceiling fixtures and luminescent curtains brighten living rooms. Entrants in these races include lighting giants such as General Electric and Osram Opto Semiconductors, along with electronics companies such as Philips and Agilent Technologies, whose joint venture, Lumileds Lighting in San Jose, CA, makes one of the world’s highest-power white LEDs.
The first results are already visible. Early white LEDs are widely sold in flashlights and head-mounted hiking lamps. Visitors to the Jefferson Memorial in Washington, DC, see a rotunda bathed in white LED light. As costs fall, efficiency rises, and the quality of the light improves, white LEDs should bust out of these niche applications and displace billions of conventional light fixtures in factories, offices, and homes. “It’s not going to just change the light bulb; it will change the lighting paradigm,” says Arpad Bergh, president of the Optoelectronics Industry Development Association, a Washington, DC-based industry group.
The new lighting technology would also dramatically cut electricity demand. According to a study commissioned by the U.S. Department of Energy, widespread adoption of next-generation white LEDs for lighting could, by 2025, slash electricity consumption by 10 percent worldwide, cutting $100 billion per year from electric bills and saving $50 billion in averted power-plant construction costs. “Lighting is a major contributor to the use of electricity. Collectively we could save half the energy we use on lighting,” says George Craford, chief technology officer at Lumileds. “Make lighting more efficient and the question of building new power plants starts to go away.”
Whiteout Conditions

White LEDs (3, 4, and 5). Brighter whites: improving white LEDs calls for brighter blue emitters (top), substrate modifications such as one-micrometer-wide ridges (center), and more efficient phosphors (bottom)
Anyone who has ever tried to change a lit light bulb has touched upon the problem with today’s lighting technology. Fingers get scorched because about 95 percent of the electricity entering an incandescent bulb is wasted as heat. Fluorescent bulbs do much better, converting 20 to 30 percent of electricity into light. But even that pales beside the potential that LEDs offer. In theory, at least, an LED can convert almost 100 percent of its electrical power into light. Developers have not yet attained such perfection, but the efficiencies of the best white-light LEDs being produced are already about halfway between those of incandescent and fluorescent fixtures.
And although conventional lighting has already pretty much maxed out in efficiency, that’s not the case with solid-state lighting. LEDs, which work on an entirely different physical principle, have at their core neither the tungsten filament of a conventional light bulb nor the gas of a fluorescent tube. Rather, they use crystalline layers that convert electrical input into optical output at a color determined by the exact composition of the material.
The colored LEDs that are the main ingredients of white-light sources have already made a dramatic impact. Take the ordinary red stoplight. In a conventional setup, one big, inefficient incandescent light bulb sits behind a red filter; the bulb guzzles about 150 watts of electricity and lasts about a year before it burns out, sometimes snarling traffic until the local highway department rushes out with a bucket truck to change it. In a growing number of traffic lights, though, a dozen or so red LEDs sit behind a clear lens, consume about 15 watts, and control traffic for five or more years before requiring replacement.
According to the California Department of Transportation, replacement of conventional traffic-light bulbs with LEDs—red, yellow, and most recently, green—has trimmed at least $10 million from the state’s annual electric bill. And nationwide, according to Strategies Unlimited, a market research firm in Mountain View, CA, LED traffic lights are becoming commonplace: as of 2002, 39 percent of red lights and 29 percent of green lights used LEDs.
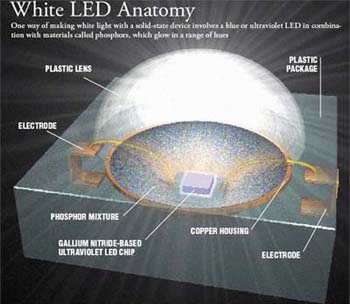
Figure 6. In this one-watt seven- millimeter device from GELcore, a one-millimeter chip emits ultraviolet light.
But coaxing LEDs to produce white light is a much tougher problem. LEDs inherently produce a single color. Producing what the eye perceives as white light requires the generation of many hues together (white itself is not actually a “color”). The easiest way to make white is to start with one LED—either blue or ultraviolet—and add materials called phosphors. The phosphors absorb those high-energy photons and generate lower energy ones, such as yellows and reds. Most of today’s white LEDs pair a blue LED with a yellow phosphor. But the resulting white lacks a full spectrum of colors and is therefore less pleasing to the eye than incandescent light; as with fluorescent tubes, which also use phosphors, skin can look a bit sickly. The newer white LEDs are better: they pair an ultraviolet-emitting LED with phosphors that put out light at a range of colors, which mix to make white (Figure 6). Any device that uses phosphors, however, loses some efficiency in the process.
Quality of lighting aside, developers must solve an even more fundamental problem before LEDs can replace the ubiquitous white light bulb: the white LEDs now being produced or developed all cost many times as much as an ordinary light bulb. “Nobody will pay 20 bucks for a light bulb–even if it lasts 500 times longer and uses half the electricity,” says Frank Steranka, vice president for research and development at Lumileds.
Overcoming the hurdles of efficiency, color, and cost is what the global race is all about. And advances on these fronts will soon drive wider adoption of LED lighting technology, says Robert Steele, director of optoelectronics at Strategies Unlimited. “In the next five years, they’ll continue penetrating a variety of specialty markets like decorative lighting and entertainment lighting. In five to 10 years, they will start to penetrate the commercial and industrial market,” Steele says. Next stop perhaps: your kitchen or living room.
Getting the LEDs Out
A white LED is only as good as its weakest component. Its performance depends on such factors as the purity of the semiconductor materials, the shape of the phosphor crystals, and even how well the lit device is able to dissipate heat. To bring white LEDs from the lab into broader markets will require improvements—and cost reductions—in all of these elements.
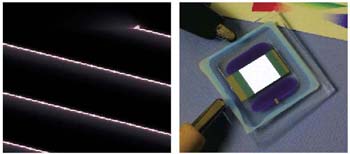
Figures 7 and 8. Glowing plastic sheets: organic LEDs could provide flexible light fixtures that hang on walls and ceilings.
The job starts in a very hot oven. The key ingredient—gallium nitride—is born from gases fed into a superheated chamber. There, molecules containing gallium and nitrogen break apart, and crystals of gallium nitride begin to grow atop a sapphire substrate (in a process akin to the making of computer chips). It takes hours to deposit the dozens of layers, each with a slightly different chemistry. And it is far from a perfect process. A single sample, depending on where it is energized, can produce light of several different colors—which is why Nakamura is poking and prodding his materials so meticulously. Moreover, subtle differences in the arrangement of atoms can result in efficiency-robbing regions that look like vertical tunnels in the material.
Improvement of efficiency calls for growing crystals without those tunnels. Researchers at Sandia National Labs in Albuquerque, NM, believe they have a simple method for doing that, thus producing brighter LEDs. They start by etching grooves into the substrate, leaving a series of thin sapphire ridges, each about one micrometer wide. These ridges act like floor joists. The gallium nitride grows atop the sapphire ridges and moves sideways out over the grooves. Experiments have shown that this method reduces the number of defects by two orders of magnitude—and boosts brightness tenfold.
Even when the material itself is efficient, the light must go where it’s needed. Much of the light produced by a typical blue LED bounces around within the structure and is wasted. Nakamura’s group at Santa Barbara is adding mirrorlike nanostructures, just 50 nanometers wide, to certain crystalline layers. Steven DenBaars, a materials scientist who works with Nakamura, says that adding the nanostructures increases by 50 percent the light that gets reflected out of the device. But because such layers have not yet been integrated into a finished LED, the full payoff is uncertain.
Size matters too. A big LED chip gives off more light than a small one. Pursuit of a bigger LED has been a focus of the work at Durham, NC-based Cree, which makes some of the brightest blue LEDs on the market and is one of several corporations that have partnered with Nakamura’s Santa Barbara lab. Last year Cree introduced an LED chip measuring 900 by 900 micrometers. It provides nine times the light-emitting surface area of the 300- by 300-micrometer chips that had been the industry standard. This expansion yields a simpler, hence cheaper, device, says Cree vice president Norbert Hiller. Lumileds, too, is developing larger chips and was able to deliver one of the world’s brightest white-light LEDs: a five-watt device that puts out as much light as a 10-watt incandescent bulb.
Advances are also needed in phosphors. That’s exactly what GELcore—a joint venture of General Electric and Somerset, NJ-based Emcore—says it has achieved. Starting with LEDs that emit ultraviolet light, researchers at GE’s Global Research Center in Niskayuna, NY, went to work improving the phosphor recipes developed for the company’s ordinary fluorescent light tubes. The result: they increased by a factor of 100 the ability of the phosphor to absorb energy, says Charles Becker, a physicist and manager of LED lighting research at GE’s research center.
Thanks to this development, GE says it is close to launching a white-light device that can produce 30 lumens per watt, a considerable improvement over the 10 to 15 lumens per watt rating that consumers are accustomed to seeing on the boxes of typical incandescent bulbs. What’s more, the device is designed to last 50,000 hours—about six times the typical incandescent’s lifetime. GELcore plans to begin selling LEDs that use this technology later this year and aims to push light output to 50 lumens per watt within two years, says Becker.
Just about every company in the white-light LED game is tackling these and other methods. One approach skips the phosphor conversion process altogether and instead produces white light by combining the output of red, green, and blue LEDs. Lumileds, for instance, is already assembling emitters of these three colors to produce backlighting for displays such as those used in cell phones and laptop computers. While bypassing the phosphor adds cost and difficulty, this technique increases efficiency.
Unfortunately, the materials of each color LED degrade at different rates. Stabilizing the white, therefore, requires sensors and electronics: as the sensor records a decline in an LED’s output, it directs the circuit to compensate by feeding more power to the chip. Steranka says that making this approach work for a low-cost general-illumination product calls for volume-driven cost reductions in such electronics, as well as more efficient individual LEDs. But the payoff would be further gains in efficiency—a goal Steranka is confident can be achieved. “People know how to do to it,” he says. “It’s not rocket science.”
Plastic light
There’s a dark horse in the white-light race: the organic light-emitting diode, or OLED. Whereas an ordinary LED makes a bright point of light, the organic variety resembles a patch of softly glowing plastic. Mass production of these products could be as simple as ink-jet printing because there is no need for the costly chip-fabrication facilities that make LEDs so expensive. Someday this technology might lead to flexible lighting fixtures that hang on walls, ceilings, and even furniture.
The main impetus for OLED development is coming from companies that seek backlighting for cheaper, brighter displays. But the technology is improving rapidly, approaching the point where it could be practical for illumination. Barely 18 months ago, GE researchers proudly touted a 2.5- by 2.5-centimeter white-light OLED that produced only 3.8 lumens per watt. Today, says Anil Duggal, manager of the light energy conversion program at GE Global Research, the company can show off a 15- by 15-centimeter prototype—about the size of a compact-disc case—with double the efficiency of the earlier OLED.
And just as Nakamura is adding nanostructures to LEDs, Duggal is adding particles to the OLED substrate to provide more chances for the light to zip from the surface of the device, rather than being absorbed internally. Employing such tricks could make OLED-based white lights competitive with fluorescent tubes within a decade, Duggal says. “There is no problem getting to high brightness, but lifetimes are still an issue, and efficiencies are an issue,” he says.
To speed development, Ewing, NJ-based Universal Display is working with a new class of OLED materials. Invented in 1999 in collaboration with the University of Southern California and Princeton University, these materials incorporate atoms of heavy metals such as platinum, surrounded by carbon-based molecules that amount to “organic shrubbery,” says Janice Mahon, vice president of technology commercialization at Universal Display. Last year the company and its academic partners used this material to build a white-light OLED prototype that achieved 11 lumens of light per watt of electricity. That device was made with a single OLED material that can emit a broad spectrum of colors. But the company is also pursuing a white-light source that relies on a combination of different materials, some emitting red, some blue, and others green. Universal Display expects to build a prototype this spring, Mahon says.
A more far-out vision for OLEDs embeds glowing specks of inorganic crystals into the lighting device. Each of these “quantum dots” is a cluster of cadmium selenide atoms one to five nanometers across. At that scale, the strange rules of quantum physics take over, and the wavelength of the emitted light depends purely on the size of the cluster. Late last year two MIT researchers—chemist Moungi Bawendi and electrical engineer Vladimir Bulovic—showed that a sprinkling of quantum dots could brighten an OLED significantly. But right now, Bulovic says, the quantum dot white OLED is only “a good proposal—it’s far away from being a proved fact.”
Regardless of whether quantum dots get their moment to shine, the future of illumination appears solid—solid-state, that is. The pitched competition across corporate and academic labs promises to upend the century-old lighting industry. In addition to Lumileds and GELCore in the United States, the leading players include Nichia and Toyoda Gosei in Japan and Osram Opto Semiconductors in Germany. “Those would be the five who have advanced the art the most,” says Steele of Strategies Unlimited.
While for the next few years these and other companies are chasing big markets in backlighting for cell phone displays and automotive instrument panels, LED developers will increasingly find paradise beyond the dashboard lights. By 2007 the LED illumination business will top $500 million, according to Strategies Unlimited—of which $135 million will be for white-light LEDs.
And that’s just a taste of what’s to come: the field is still wide open for technology breakthroughs. “It is always possible that someone from left field is going to come in and do something dramatic,” Steele says. Indeed, he and other industry observers agree, the great number of players pursuing solid-state lighting technology—and the great theoretical potential of LEDs—makes major advances in white-light LED illumination inevitable. It’s only a question of who will make that next breakthrough.
In the test room at Nakamura’s Santa Barbara laboratory, grad student John Kaeding describes the researchers’ efforts by citing the adage about 10,000 monkeys pounding on 10,000 typewriters and eventually producing a work of Shakespeare. That old saw is new to Nakamura, but when the light pioneer absorbs the point, he nods vigorously: “Yes, yes,” he says. With so many labs attacking the technology from so many angles, he believes that someday, somewhere, a researcher will open an oven and pull out a material that can emit a blue glow of unmatched brilliance—radically advancing the way we light our world.
No wonder the shades are drawn on Nakamura’s windows.
Copyright © 2003, David Talbot. All Rights Reserved. Reprinted by permission ofTechnology Review, An MIT Enterprise. (http://www.technologyreview.com).









Find Us on Socials