Accreditation is the means by which an independent third party, an accreditation body, provides formal recognition that an organization is competent to carry out specific tasks. When accreditation services are provided by an accreditation body that is a signatory of the International Laboratory Accreditation Cooperation (ILAC) mutual recognition arrangement (MRA) or International Accreditation Forum (IAF) multi-lateral arrangement (MLA), it provides assurance to stakeholders, such as regulatory authorities, that the accredited organization operates in accordance with specified criteria.
ILAC MRA and IAF MLA signatories comply with the requirements ISO/IEC 17011, Conformity assessment –General requirements for accreditation bodies accrediting conformity assessment bodies. Conformance to these requirements is verified through rigorous on-site evaluation by other members of the international (ILAC or IAF) community. Accreditation bodies are admitted to the ILAC MRA and/or IAF MLA for a specific capability, for example, as an accreditor for testing or inspection organizations.
The IAF MLA and ILAC MRA are internationally recognized forms of approval; signatories have demonstrated their compliance with specified standards and requirements. Accreditation by a signatory of the ILAC MRA and/or IAF MLA provides assurance that decisions are based on reliable results, thus minimizing risk. Many specifiers, such as regulatory authorities have acknowledged the importance of credible accreditation programs. Accreditation and the MLA/MRA process help regulators meet their legislated responsibilities by providing assurance that testing, inspection, or evaluation results are issued by an organization whose technical competence and compliance with specified criteria has been verified by an independent third party.
A number of government agencies in the United States, such as Department of Energy (DoE), Department of Defense (DoD), Consumer Product Safety Commission (CPSC), and Environmental Protection Agency (EPA), and many others have mandated accreditation by an internationally recognized accrediting body for their programs. These and many other federal and state agencies rely on the accreditation process as a means of confirming technical competence and conformance to specific requirements.
Accreditation bodies use internationally established techniques and procedures to assess all aspects relevant to an organization’s ability to produce consistently reliable, technically competent, and impartial evaluation results, including consideration of the following:
- Technical competence of staff (including qualifications, training, and experience)
- Appropriateness of evaluation methods
- Use of suitable equipment (properly calibrated and maintained)
- Safeguards to ensure impartiality and confidentiality
- Code of conduct and processes for working safely
- Effective quality assurance procedures
Although there is flexibility within the constraints of the ISO/IEC 17011 standard for an accreditation body to design its accreditation process, some aspects are mandatory.
The accreditation process typically begins when an interested party provides the accreditation body with information on the desired scope of accreditation, size of the organization, number of employees, and locations where activities to be covered under the scope of accreditation will be conducted. Based on this information, the accreditation body determines the composition of the assessment team and estimates the time required to adequately evaluate conformance with requirements of the relevant standards (such as NFPA 790).
The applicant is required to submit a completed application package consisting of:
- Completed application form, including all relevant locations to be covered by the accreditation
- Quality management system documentation, including the organization’s quality manual and copies of procedures and work instructions
- Organizational structure, specifically noting the persons or organizations responsible for assigning the property values of all artifact types related to the application for accreditation
- List of all relevant experts, if applicable
As part of the application process, the applicant submits information about the desired scope of accreditation. The scope defines the specific evaluation capabilities for which the accreditation will apply. A field evaluation body (FEB) could apply for all or one of the Electrical Product Groups identified in Annex C of NFPA 790. For regulatory authorities seeking competent FEBs, accreditation to NFPA 790 for the required scope of evaluation provides the needed assurance.
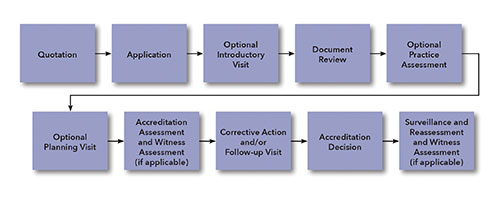
Document review is conducted to verify that an applicant has documented all management system requirements specified in the relevant criteria, such as NFPA 790, and any other applicable requirements. Additional requirements could include, for example, those mandated by a specific regulatory authority or industry.
Depending on the severity of gaps identified, the applicant may be required to address issues before the on-site assessment is conducted.
During the on-site accreditation assessment, through interviews and review of records and other objective evidence, the assessment team verifies technical competence and implementation of the quality management system. The applicant is required to provide corrective action for all identified deficiencies. Only after all identified issues have been addressed can the accreditation decision process begin. The assessment team makes a recommendation on whether the applicant meets all applicable requirements.
If necessary, a follow-up visit is scheduled prior to the accreditation decision.
To ensure that the accreditation decision is impartial, members of the assessment team do not take part in the initial accreditation decision. The designated decision maker is responsible for reviewing the assessment team’s recommendation and ensuring that all accreditation requirements have been met by the applicant and are properly documented before granting accreditation.
A certificate and scope of accreditation are issued only after a favorable accreditation decision.
Once accredited, organizations are re-assessed regularly to ensure continued compliance with requirements, and to confirm that the required standard of operation is being maintained. Surveillance and reassessment typically consist of biennial on-site assessments and surveillance during alternating years.
Any changes occurring after initial accreditation, such as suspension for all or part of the scope of accreditation, are publically displayed on the accrediting agency’s website.
The above diagram illustrates accreditation process for ANAB’s field evaluation program.
As more field evaluation organizations compete for the ability to evaluate equipment in various jurisdictions around the United States, accreditation is the means to assure authorities having jurisdiction that work is conducted by a technically competent FEB. Accreditation also ensures a uniform approach in the qualification and assessment of field evaluation agencies across the nation.
Accreditation demonstrates that an organization has the necessary technical competence and has met and continues to meet a specific set of operational requirements.











Find Us on Socials